Palmitic and Stearic Acids: Maintaining Milk Fluidity
Posted by Dr. Jim Lofton on Aug 3, 2022 11:31:19 AM
 The recent interest in feeding highly concentrated palmitic acid (C16:0) to lactating dairy cows for the purpose of improving milk fat test and yield has caused researchers and consultants alike to search for information to aid in making informed decisions on long chain fatty acid (LCFA) supplementation. The mammary gland is constantly combining fatty acids (FA) of different melting points to maintain milk fluidity. The melting point of C16:0, stearic acid (C18:0), and oleic acid (C18:1) is 145° F, 157° F, and 56° F respectively. The cows’ normal body temperature is 101.5° F. 200 When the mammary gland is presented with plasma triglycerides (TG) rich 150 in C16:0, the tissue blends lower melting point FA in the sn-3 position on 100 the TG which is preferentially reserved for these FA as shown in Table 1.
The recent interest in feeding highly concentrated palmitic acid (C16:0) to lactating dairy cows for the purpose of improving milk fat test and yield has caused researchers and consultants alike to search for information to aid in making informed decisions on long chain fatty acid (LCFA) supplementation. The mammary gland is constantly combining fatty acids (FA) of different melting points to maintain milk fluidity. The melting point of C16:0, stearic acid (C18:0), and oleic acid (C18:1) is 145° F, 157° F, and 56° F respectively. The cows’ normal body temperature is 101.5° F. 200 When the mammary gland is presented with plasma triglycerides (TG) rich 150 in C16:0, the tissue blends lower melting point FA in the sn-3 position on 100 the TG which is preferentially reserved for these FA as shown in Table 1.
Table 1. The molar % of milk fatty acids at the 3 different positions on the milk fat TG and their corresponding melting points. Adapted from Jenson, 2000.
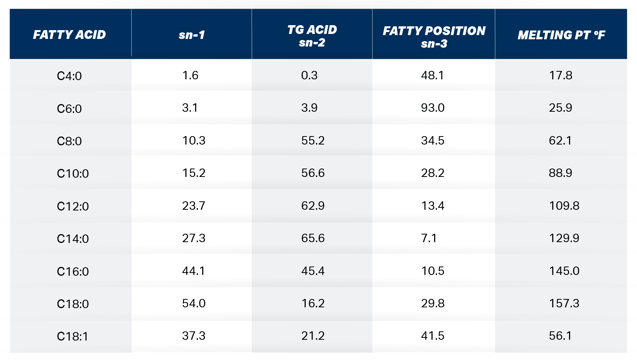
C16:0 at high levels of intake pose a problem for the cow with respect to milk FA content. As observed in the 7 recent production trials, only 15-20% of the added C16:0 intake is incorporated into milk fat. There appears to be a mechanism in place that limits the amount of C16:0 and other high melting point FA in milk fat. Timmon and Patton 1988 reported in their landmark publication regarding milk fluidity that the small differences in milk FA, consistently detected, seem important for a number of reasons. It is notable that the fatty acids involved are crucial to the liquidity of milk fat. It is a liquid at body temperature presumably of necessity for its synthesis and secretion by the lactating cell, as well as for efficient utilization by the offspring. The principal means of assuring this liquidity is the incorporation of C18:1 (mp 56 F), which is produced from C18:0 (mp 157 F) in bovine mammary tissue, and of short chain FA (C4:0-C10:0, mp -17 to +89 F) into TGs destined for milk fat globules. The conversion of C18:0 to C18:1 in lactating bovine tissue is accomplished by the microsomal enzyme, stearyl-CoA desaturase. It is evident that the membrane fluidity-desaturase activity relationship could provide a basic control mechanism for maintaining liquidity of accumulating fat droplets.
Figure 1. Relative incorporation of acetate by exogenous fatty acids in mammary tissue in uM. Adapted from Hansen and Knudsen 1987
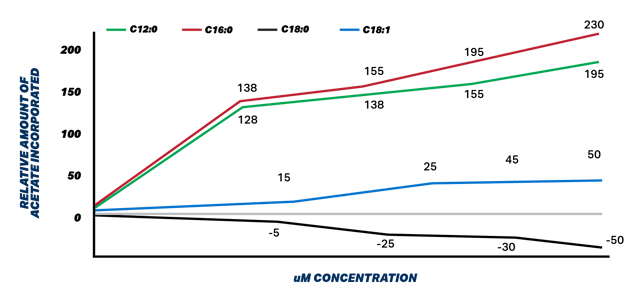
Figure 1 illustrates the stimulatory effects that palmitic and lauric acids have on denovo synthesis as measured by acetate incorporation into milk TGs. These researchers washed mammary tissue on any FA and added different FA to determine their effect on denovo synthesis. The highest melting point FA are causing increased denovo synthesis activity to accommodate need to combine lower melting point FA into milk fat TGs to maintain milk fluidity.
The composition of a LCFA supplementation appears to be very important to milk fat production and its FA composition. LCFA supplements high in C16:0 will require either adequate small and medium chain FA from denovo synthesis to balance the fluidity, or adequate C18:0 that can be readily converted into C18:1 for that purpose. In the presence of milk fat depressing situations that severely limit the small and medium chain low melting point FA availability, more C16:0 will aggravate the situation since it will be limited for use in milk fat synthesis. A balance of C16:0 and C18:0 at or near a 1:1 ratio will be superior in maximizing milk fat production.
References:
Loften, J. R. et al., 2014. Palmitic and Stearic Acid Utilization and Metabolism in Lactating Dairy Cows. J Dairy Sci 97
Timmen, H., and S. Patton. 1988. Milk Fat Globules: Fatty Acid Composition, Size and in vivo Regulation of Fat Liquidity. Lipids. Vol. 23. No. 7. 685-689
Jensen, R. G., 2002. The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci. 85:295-350.
Hansen, H. O., and J. Knudsen. 1987. Effect of exogenous long-chain fatty acids on individual fatty acid synthesis by dispersed ruminant mammary gland cells. J. Dairy Sci. 70:1350-1354.
